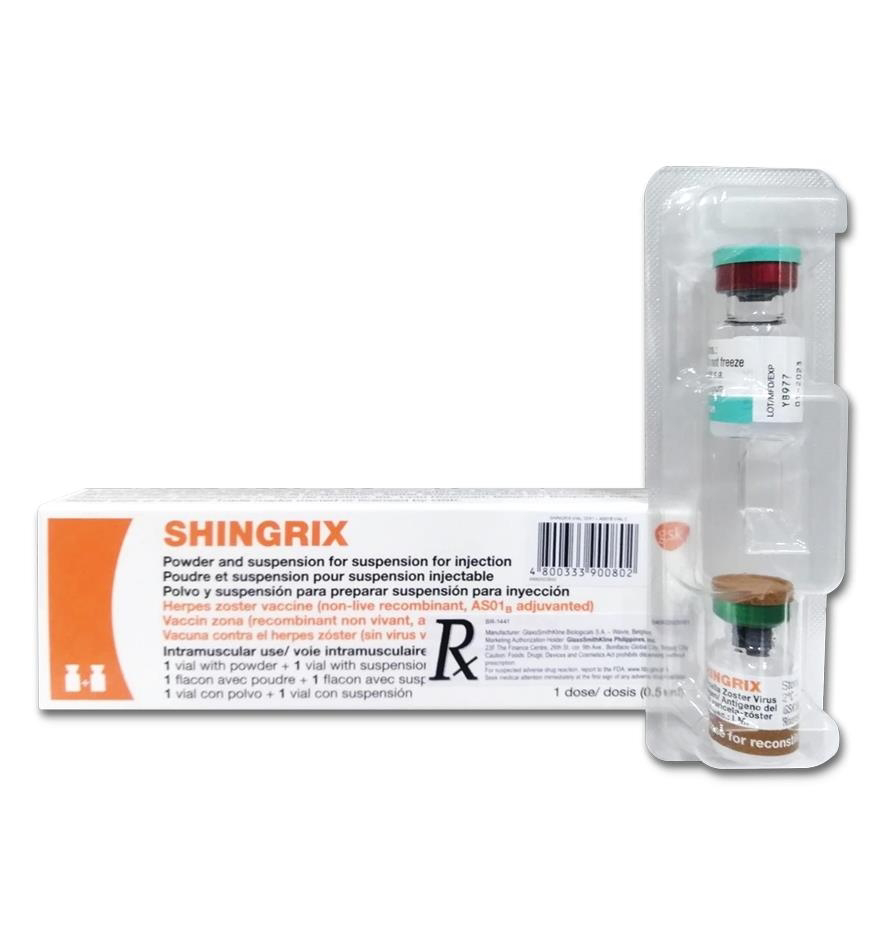
GlaxoSmithKline Pharmaceutical Sdn Bhd (GSK) has announced the launch of its Herpes Zoster vaccine, Shingrix (MAL24056002ARZ), also known as Recombinant Zoster Vaccine, Adjuvanted (RZV) in Malaysia for the prevention of shingles (herpes zoster, HZ) and post-herpetic neuralgia (PHN) in adults aged 50 years and over.i RZV is a non-live, recombinant subunit adjuvanted vaccine given intramuscularly in two doses.
RZV will initially be available in Malaysia to adults aged 50 and over and those aged 18 and over who are at increased risk of HZ.
Shingles is caused by the reactivation of the varicella zoster virus (VZV), the same virus that causes chickenpox.ii As people age, the cells in the immune system lose the ability to mount a strong and effective response to infection, increasing the risk of developing shingles.iii The disease can cause unbearable pain and, in some cases, people can also develop post-herpetic neuralgia (PHN) a long-lasting nerve pain following shingles.
RZV is a vaccine designed to prevent shingles in adults aged 50 years and over and 18 years and over who are at increased risk in countries where the indication for this population has been approved.
RZV is also approved for adults aged 18 and over who are at increased risk of HZ. Immunocompromised individuals are at greater risk of shingles and associated complications and RZV is a shingles vaccine approved for this at-risk patient population.
In an analysis of the pivotal efficacy studies, ZOE-50, RZV demonstrated vaccine efficacy of up to 97% in adults 50 years and above, over a follow-up period of approximately three years.
Recent interim data demonstrated overall vaccine efficacy of RZV of greater than 80% over the primary follow-up period approximately six to 10 years after initial vaccination in people 50 years and over.
Dr. Alap Gandhi, Country Medical Director of GlaxoSmithKline Pharmaceutical Sdn Bhd (GSK) Malaysia said: “Shingles is a disease that can cause excruciating pain and is caused by the reactivation of the varicella zoster virus. RZV has been designed to boost the immune response to the virus in people aged 50 and over or in those with immunocompromised conditions.”
He concluded, “We are pleased that people in Malaysia will now have access to the RZV vaccine to help reduce the burden of this painful disease.”
RZV is an approved shingles vaccine to combine a non-live antigen with GSK’s adjuvant and may help overcome the natural age-related decline in immunity that contributes to the challenge of protecting adults aged 50 and over from this disease.
RZV has resulted in positive vaccination recommendations. In Malaysia, RZV is recommended by Guideline for Adult Immunization by Malaysia Society of Infectious Diseases and Chemotherapy for persons who are 50 years and above and for immunosuppressed patients 18 years and above.
With the launch of RZV in Malaysia, GSK hopes to continue to play a part in the betterment of public health in Malaysia.
About Shingles
Shingles is caused by varicella zoster virus (VZV), the same virus that causes chickenpox. Adults aged 50 and over may have the VZV dormant in their nervous system, that may reactivate with advancing age.
As people age, the cells in the immune system lose the ability to mount a strong and effective response to infection, increasing the risk of developing shingles.
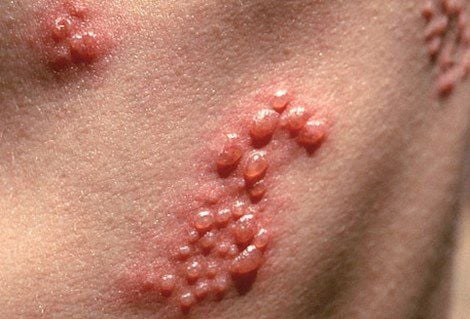
Shingles typically presents as a rash, with painful blisters across the chest, abdomen or face. The pain is often described as aching, burning, stabbing or shock-like. Following the rash, a person can also experience post-herpetic neuralgia (PHN), pain lasting from at least three months up to several years. PHN is the most common complication of shingles, occurring in up to 10-20 percent of all shingles cases depending on their age.
About Shingrix
Shingrix [Herpes Zoster vaccine (recombinant, AS01Bix adjuvanted)] is a non-live, recombinant subunit vaccine indicated for the prevention of shingles (herpes zoster) and, in some markets, for post-herpetic neuralgia (PHN), in adults 50 years of age and over.
Approval of RZV follows a comprehensive Phase III clinical trial program evaluating its efficacy, safety, and immunogenicity in more than 30,000 people. The most common local side effects reported in the clinical trials were pain, redness and swelling at the injection site. The majority were mild to moderate in intensity and transient, generally lasting less than three days.
The indication for RZV also includes the prevention of HZ in adults aged 18 and over who are or will be at increased risk of herpes zoster due to immunodeficiency or immunosuppression caused by known disease or therapy.
RZV, given intramuscularly in two doses, was designed to enhance the immune response of a declining or compromised immune system.
About GSK
GSK is a global biopharma company with the ambition and purpose to unite science, technology and talent to get ahead of disease together. GSK aim to positively impact the health of 2.5 billion people over the next 10 years. Their bold ambitions for patients are reflected in new commitments to growth and a step-change in performance. They have been operating in Malaysia for over 65 years, since starting their first operations in October 1958. In the last six decades, GSK Malaysia has grown substantially both in its infrastructure, business operations as well as talent base. Currently operating with over 400 employees spread across their two business entities – Pharmaceuticals & Vaccines and Business Service Centre.






No comments:
Post a Comment
What do you think of my blog???